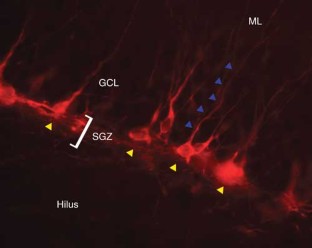
BrdU assay for neurogenesis in rodents
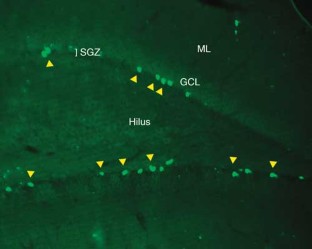
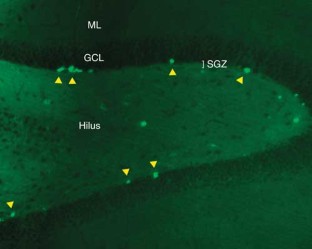
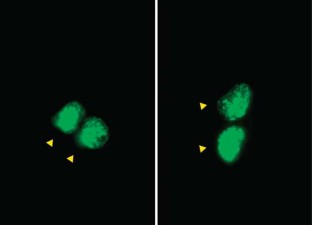
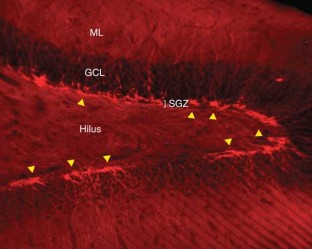
Neurogenesis within the adult central nervous system is demonstrated using an exogenous cell tracer, 5'-bromo-2'-deoxyuridine (BrdU), in combination with endogenous neuronal markers. Specific primary antibodies raised against these markers are widely available and their visualization is possible with the use of fluorescently tagged secondary antibodies. BrdU is a thymidine analog that incorporates into dividing cells during DNA synthesis. Once incorporated into the new DNA, BrdU will remain in place and be passed down to daughter cells following division. Typically, BrdU is injected intraperitoneally. Different survival times required by the desired experimental time-line will yield data on specific phases of neurogenesis: proliferation, differentiation and maturation. One of the drawbacks of using BrdU is the dependence on a stressful injection procedure and uncertain penetration of the targeted cells with a uniform concentration of the compound. Thus, for experiments requiring measurements of cell proliferation, Ki67 can be used as an acceptable alternative. The protocol takes 3–5 d, allowing for sectioning and staining.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
265,23 € per year
only 22,10 € per issue
Buy this article
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Steps towards standardized quantification of adult neurogenesis
Article Open access 26 August 2020

Unraveling human adult hippocampal neurogenesis
Article 08 January 2020

A cell fate decision map reveals abundant direct neurogenesis bypassing intermediate progenitors in the human developing neocortex
Article Open access 28 March 2024
References
- Cameron, H.A. & McKay, R.D. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J. Comp. Neurol.435, 406–417 (2001). ArticleCASGoogle Scholar
- Kempermann, G. et al. More hippocampal neurons in adult mice living in an enriched environment. Nature386, 493–495 (1997). ArticleCASGoogle Scholar
- Corotto, F.S. et al. Neurogenesis persists in the subependymal layer of the adult mouse brain. Neurosci. Lett.12, 111–114 (1993). ArticleGoogle Scholar
- Gratzner, H.G. Monoclonal antibody to 5-bromo- and 5-iododeoxyuridine: a new reagent for detection of DNA replication. Science218, 474–475 (1982). ArticleCASGoogle Scholar
- Kuhn, H.G. et al. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J. Neurosci.15, 2027–2033 (1996). ArticleGoogle Scholar
- Nowakowski, R.S. et al. Bromodeoxyuridine immunohistochemical determination of the lengths of the cell cycle and the DNA-synthetic phase for an anatomically defined population. J. Neurocytol.18, 311–318 (1989). ArticleCASGoogle Scholar
- Brown, D.C. & Gatter, K.C. Ki67 protein: the immaculate deception? Histopathology40, 2–11 (2002). ArticleCASGoogle Scholar
- Endl, E. & Gerdes, J. The Ki-67 protein: fascinating forms and an unknown function. Exp. Cell Res.257, 231–237 (2000). ArticleCASGoogle Scholar
- Hofmann, K. & Bucher, P. The rsp5-domain is shared by proteins of diverse functions. FEBS Lett.358, 153–157 (1995). ArticleCASGoogle Scholar
- Kee, N. et al. The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J. Neurosci. Meth.115, 97–105 (2002). ArticleCASGoogle Scholar
- Schluter, C. et al. The cell proliferation-associated antigen of antibody Ki-67: a very large, ubiquitous nuclear protein with numerous repeated elements, representing a new kind of cell cycle-maintaining proteins. J. Cell Biol.123, 513–522 (1993). ArticleCASGoogle Scholar
- Scholzen, T. & Gerdes, J. The Ki-67 protein: from the known and the unknown. J. Cell Physiol.182, 311–322 (2000). ArticleCASGoogle Scholar
- Meyer, G. et al. Selective expression of doublecortin and LIS1 in developing human cortex suggests unique modes of neuronal movement. Cereb. Cortex12, 1225–1236 (2002). ArticleGoogle Scholar
- Schaar, B.T. et al. Doublecortin microtubule affinity is regulated by a balance of kinases and phosphatases activity at the leading edge of migrating neurons. Neuron41, 203–213 (2004). ArticleCASGoogle Scholar
- Brown, J.P. et al. Transient expression of doublecortin during adult neurogenesis. J. Comp. Neurol.467, 1–10 (2003). ArticleCASGoogle Scholar
- McDonald, H.Y. & Wojtowicz, J.M. Dynamics of neurogenesis in the dentate gyrus of adult rats. Neurosci. Lett.385, 70–75 (2005). ArticleCASGoogle Scholar
- Quinn, C.C. et al. A family of proteins implicated in axon guidance and outgrowth. J. Neurobiol.41, 158–164 (1999). ArticleCASGoogle Scholar
- Seki, T. & Arai, Y. Temporal and spacial relationships between PSA-NCAM-expressing, newly generated granule cells, and radial glia-like cells in the adult dentate gyrus. J. Comp. Neurol.410, 503–513 (1999). ArticleCASGoogle Scholar
- Mullen, R.J. et al. NeuN, a neuronal specific nuclear protein in vertebrates. Development116, 201–211 (1992). CASPubMedGoogle Scholar
- Baimbridge, K.G. & Miller, J.J. Immunohistochemical localization of calcium-binding protein in the cerebellum, hippocampal formation and olfactory bulb of the rat. Brain Res.245, 223–229 (1982). ArticleCASGoogle Scholar
- Celio, M.R. Calbindin D-28k and parvalbumin in the rat nervous system. Neuroscience35, 375–475 (1990). ArticleCASGoogle Scholar
- Kempermann, G. et al. Milestones of neuronal development in the adult hippocampus. TINS27, 446–452 (2004). Google Scholar
- Unal-Cevik, I. et al. Loss of NeuN immunoreactivity after cerebral ischemia does not indicate neuronal cell loss: a cautionary note. Brain Res.1015, 169–174 (2004). ArticleCASGoogle Scholar
- Magloczky, S. et al. Loss of calbindin-D28k immunoreactivity from dentate granule cells in human temporal lobe epilepsy. Neuroscience76, 377–385 (1997). ArticleCASGoogle Scholar
- Wang, S. et al. Electrophysiological correlates of neural plasticity compensating for ischemia-induced damage in the hippocampus. Exp. Brain Res.165, 250–260 (2005). ArticleGoogle Scholar
- West, M.J. et al. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator procedure. Anat. Rec.231, 482–497 (1991). ArticleCASGoogle Scholar
- Anisimov, V.N. The sole DNA damage induced by bromodeoxyuridine is sufficient for initiation of both aging and carcinogenesis. Ann. NY Acad. Sci.719, 494–501 (1994). ArticleCASGoogle Scholar
- Kolb, B. et al. Embryonic and postnatal injections of bromodeoxyuridine produce age-dependent morphological and behavioral abnormalities. J. Neurosci.19, 2337–2346 (1999). ArticleCASGoogle Scholar
Acknowledgements
We thank CIHR (Canada) for financial support, and present and past members of Wojtowicz' lab for their contributions.
Author information
Authors and Affiliations
- Department of Physiology, University of Toronto, Toronto, M5S 1A8, Ontario, Canada J Martin Wojtowicz
- Program in Neuroscience and Mental Health, The Hospital for Sick Children, Toronto, M5G 1X8, Canada Nohjin Kee
- J Martin Wojtowicz









